Abstract
Cancer heterogeneity and development of resistance is the main limiting factor for the management and treatment of the disease. However, major technological innovations in precision medicine and immune-based therapies have renewed faith in having a cure for different types of cancers. Classical cancer treatment options include chemotherapy, radiotherapy, and immunotherapy. Emergence of novel tumor targeting bacteria could open up new therapeutic avenues. Bacteriotherapy alone or in conjunction with classical cancer therapies has given promising results on local tumor regression and distant metastasis. Moreover, bacteria exhibit direct anti-cancer effects that subsequently aid in the activation of innate and adaptive anti-tumor immune responses. Overall, genetically reprogrammed bacterial vectors holds great potential for the specific targeting of cancers as delivery vehicles. In this review article, we have reviewed the therapeutic potential of bacteriotherapy as monotherapy or combination therapy and discussed its benefits, challenges, and future directions.
Keywords: Cancer; bacteriotherapy; chemotherapy; radiotherapy; immunotherapy.
1. Introduction
Cancer is a major health concern and one of the leading causes of death. According to WHO estimates, 19.3 million new cases of cancer and nearly ten million cancer deaths will occur in 2020 (1). Tobacco use, exposure to chemicals or other highly mutagenic agents, and infection with a microbe like HPV or HCV and HBV are all known to influence cancer development (2)– (5). Most of the malignant neoplasms share similar etiopathogenecity but diverge on tissue of origin, histopathologic attributes, immunologic properties, intertumoral and intratumoral heterogeneity (6).
Cancer initiation and progression is a complicated multihit process involving a variety of functional and genetic abnormalities. Cancer disease is characterized by unchecked cell growth and proliferation that often spreads to secondary sites in the body termed as metastasis (7). Cancer tumor microenvironment (TME) consists of a variety of stromal and immune cells. Immune cells commonly present in the TME include macrophages, mast cells, myeloidâ€derived suppressor cells (MDSCs), dendritic cells (DCs), NK cells, and T cells. Over the last century, significant advances in cancer treatment have been made. On the market, there are dozens of cytotoxic anticancer agents. For patient prognosis and treatment, traditional therapies such as surgery, chemotherapy, radiotherapy, immunotherapy, and combination therapy are used. Conventional cancer therapies often eradicate cancer cells at the expense of normal tissue damage and its use causes systemic toxicities (8). These practices often have a number of adverse effects and limited penetrance. Therefore, efforts are underway to establish a treatment strategy, which is devoid of any negative outcomes.
Experiments and clinical studies were conducted over the last several years to assess microbes as a potential therapeutic option for cancer control
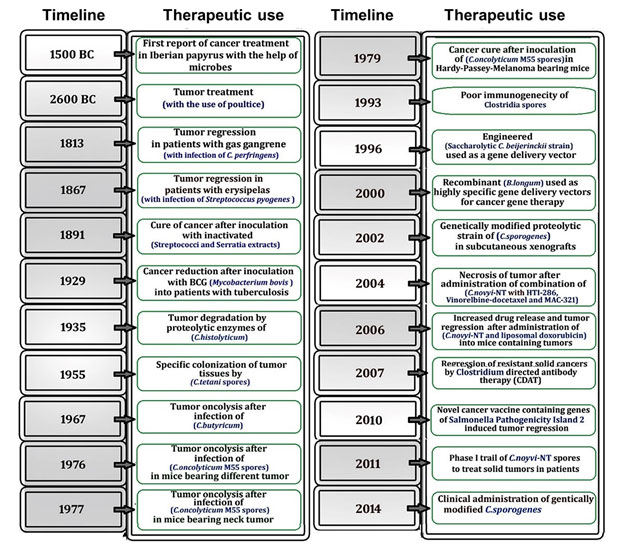
Figure 1. A timeline depicting the historical use of bacteria for cancer therapy. Intriguing research findings over the last few decades suggest that bacterial use could be an effective cancer treatment strategy.
|
(9)– (12). For instance, one good example is the use of Bacillus Calmette–Guerin (BCG) for the treatment of bladder cancer (13). The immunomodulatory activity of BCG is assumed to produce anti-cancer effects (9, 14). In addition, azurin, a bacterial redox protein, has been shown to cause apoptosis in melanoma cells. expressing functional p53 tumor suppressor protein (15). Azurin interacts with p53 and forms a complex that stabilizes p53, which leads to the induction of p53-mediated apoptosis in melanoma cells (15, 16). p53 is a well-established tumor suppressor protein (17). Like p53, its family members, p63 and p73 are also tumor suppressor genes and promote senescence and apoptosis (17, 18).
Novel therapeutic strategies using live or genetically modified microbes to target the localized disease might be an effective way of immune system activation. Several microorganisms have been tested against cancers and some are going through phase II and phase III clinical trials (clinicaltrials.gov/ct2/show/NCT00004988, clinicaltrials.gov/ct2/show/NCT03358511,
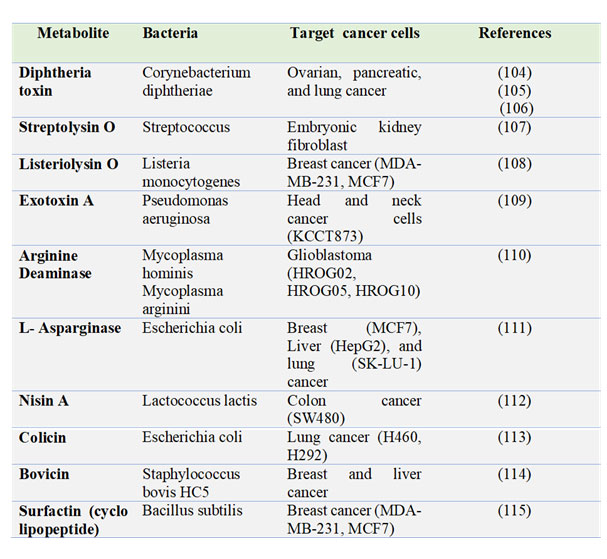
Table 1: Summary of bacteria-derived oncolytic molecules targeting different human cancers.
|
clinicaltrials.gov/ct2/show/NCT01118819). Historical scientific observations had revealed that bacterial infections enhance anti-tumor effects. In addition, bacteria can effectively invade hypoxic areas in the tumor tissue, which is reported to provide resistance to conventional cancer therapies. Furthermore, a few studies have shown that parasitic microbes can inhibit tumor progression (19)– (21). One such parasite is Plasmodium, which causes the malarial disease in humans (22)– (25). Several studies have found that malaria parasites can kill cancer cells by activating the host's innate and adaptive immune systems (19, 26, 27).
Due to the advancements in radiation therapies in the nineteenth century, the attention of the scientific community was diverted from microbe-based anti-cancer therapies (9). However, in the 1990s the concept and understanding of the tumor microenvironment (TME) and DNA recombinant technology was broadly improved. More potent bacterial strains were developed and tested in animals for their anti-tumor potential. Bacteria can also be used as vectors to execute tumoricidal and immunomodulatory activities for the destruction of cancer cells. However, the major hurdle in developing bacteria as promising anti-tumor agents is the toxicities associated with their infection. Although it is unlikely to have a cure for cancer anytime soon, innovative efforts will continue to leverage the power of bacteria for improving the cancer treatment. In this review article, we discuss the current state and future prospects for the using bacteria in anti-cancer therapy to advance cancer treatment.
2. Role of microbes in cancer regression: historical aspects
The origin of microbial therapy against cancer has been there for several centuries (Fig. 1). The first report describing the role of microorganisms in cancer treatment was documented by Iberian papyrus (1550 BC), followed by Egyptian pharaoh Imhotep (2600 BC) (28). Various forms of cancer immunotherapy became popular in the 17th and 18th centuries. In 1813, Vautier observed tumor regression in patients with gas gangrene after infection of C. perfringens (11). Similarly, in 1867, the German physician Wilhelm Busch described a case of cancer remission in a patient who had erysipelas, now known as Streptococcus pyogenes (29). In the late 19th century, William B. Coley, who posited the hypothesis that Streptococcal bacterial infections triggered the immune system to restrict the tumor growth, leading to the discovery of an important bacteria-based therapeutic strategy against cancer. Coley developed Coley's toxin, a mixture of lysates derived from heat-killed Streptococcus pyogenes bacteria, which is recognized as the first attempt of cancer immunotherapy (30). This fortuitous benefit of concurrent infection of bacterial pathogens in tumors prompted the investigation of microbial use for targeting cancer.
Bacteria produces a number of anti-cancer molecules including toxins, enzymes, bacteriocins, and biosurfactants (Table 1) Moreover, the most promising clinical application of an attenuated Mycobacterium bovis, Bacillus Calmette-Guerin (BCG), for the treatment of bladder cancer was established by Morales, Eidinger and Bruce in 1976 (31). BCG, a tuberculosis vaccine, is the only bacterial agent approved by the FDA. However, a number of patients fail to respond to BCG therapy, it is recommended as the standard of care for high-risk urinary bladder tumors in conjunction with immunotherapy (32).
3. Aiming for the perfect bacterium for cancer therapy
Tumor-bacterial biological interactions are important in anti-cancer properties that are either direct or immune-mediated. Bacteria-associated molecular factors that influence the magnitude of anti-tumor responses include tumor chemotaxis, cytotoxic potential, motility, invasive capacity, composition, and abundance of pathogen-associated molecular patterns (PAMPs) (12, 33)– (37). Current cancer therapies are unable to penetrate deeper in the hypoxic tumor microenvironment, however, certain bacteria can steadily migrate deeper in such areas. To improve the targeting of tumor cells, two approaches are commonly used. First is genetic engineering of the bacteria to display tumor-specific molecules on their surface. For instance, αvβ3 integrin, is highly expressed on tumors, Salmonella ppGpp-deficient strain SHJ2037 was modified to express the integrin binding peptide Arg-Gly-Asp (RGD) on the bacterial cell surface to facilitate the specific targeting of cancer cells (38). Second method involves the genetic modification of the bacteria to express a prodrug converting enzyme herpes simplex virus thymidine kinase (HSV-TK) that can activate ganciclovir (GCV) to produce anti-tumor effects (39). The standard that allows the microbe to turn out to be an appropriate tool for cancer therapy includes nontoxic to host, selective for tumor, and deeper penetration of TME. Additionally, it should not trigger an early immune response (40). Salmonella, Mycobacterium, Streptococcus, Clostridium, Listeria, Bifidobacterium, and Shigella are among the bacteria commonly used in tumor-targeting therapies.
3.1 Mycobacterium: An obligate anaerobic, facultative intracellular, non-motile bacterium that infects cattle and has the potential to cause similar disease in humans (41). At the beginning of the twentieth century, there were a few evidences that highlighted a correlation between the occurrence of tuberculosis and tumor regression (42). The attenuated Mycobacterium bovis (BCG) was widely used to treat superficial bladder cancer (43). BCG therapy was originally designed as a tuberculosis vaccine and has since evolved into one of the most successful adjuvant therapy. Moreover, in several parts of the world, it became the standard of care for superficial bladder cancer. The mechanism of action of BCG is to stimulate the body's own innate and adaptive immune responses. The most commonly used intravesical immunotherapy for the treatment of early-stage bladder cancer is BCG (41). It helps in decreasing tumor growth and its distant spread in the body, as well as prevents the relapse of cancer.
3.2 Streptococcus: Streptococcus, a gram-positive, facultative anaerobic bacterium, which was initially utilised in the treatment of bone sarcoma by Dr. William Coley (30). Microbes also harness the host immune system for mediating their indirect anti-tumor effects. Activated immune cells disrupt the neoplasm and inhibit the progression of cancer. In addition, it reduces the lymphangioma (44). After even first day of administration, the amount of immune cells including neutrophils, macrophages, as well as other lymphocytes, rapidly increase in the circulation (30, 45). The anti-cancer effects of bacterial administration are not immediately observed, but rather appear after a few months of treatment. Streptococcal strains are able to stimulate the immune system of the host to attack the tumor cells (46). In particular, it activates the innate immune system that further promotes the production of TNF-, IFNγ-, IL-12, and other inflammatory mediators (46). Overall, Streptococcus spp. mediates an important role in integrating complicated immune cell responses to enhance host's anti-tumor activity.
3.3 Clostridium: Clostridium, an obligate or facultative anaerobe is another promising bacterium for cancer treatment. They were preferred for cancer therapy because they thrive in environments with little or no oxygen (hypoxia). Blood vessels transport oxygen to the cells, which only penetrate the surface of tumors. As a result, oxygen transfer into the tumor is impaired, resulting in hypoxia. The anaerobic milieu favours the growth and development of anaerobic microbes including Clostridium spp., Salmonella spp., Bifidobacterium spp., or Listeria spp. (47, 48). The primary advantage of using microbes over conventional cancer therapies is that they grow right inside the tumor areas and specifically kill cancer cells as opposed to chemotherapies, which affects the body systemically (48, 49). Clostridium novyi is one of the most extensively studied bacterium. Deletion of its α-toxin gene, enables Clostridium novyi to colonize the tumor with less to moderate negative effects (33). It was used in experimental models for gliomas, colorectal cancer (50), and sarcomas (51) to observe its role in tumor cell death by analyzing its role in selective colonization, immune cell infiltration, and elevating their effector functions (51). Clostridium spores are less immunogenic that allows them to colonize multiple organs when administered systemically in the body (52). However, they stimulate an inflammatory immune response once they germinate in the body. This causes increased infiltration of immune cells that also produce oncolytic effects (53). These strains have been genetically engineered as vectors and vehicles to enhance the production of cytokines including TNF-α, IL-12, and IL-2 (54)– (56). Furthermore, C. novyi-NT and C. sporogenes have been shown to increase the antibody secretion against hypoxia inducing factor-1 (HIF-1) (57).
3.4 Listeria: Listeria, a gram positive, facultative intracellular bacterium, which can be used to inhibit the tumor progression or metastasis in an immune-privileged microenvironment. As it selectively colonizes the tumor and helps in their elimination through ROS production (58). Additionally, this bacterium tends to show immunomodulatory activities as it decreases the recruitment of T-reg cells in tumor microenvironment (59). Because it only infects APCs, this bacterium is thought to be a valuable immunostimulant or immunotherapeutic agent. Listeria infection strongly activates innate immunity and promotes the production of several pro-inflammatory cytokines (60). After phagocytic internalization, Listeria monocytogenes (Lm) escapes its clearance in the phagolysosomes by producing a virulence protein known as listeriolysin O (LLO) (61). LLO acts like a hemolysin, perforates the phagosomes, and comes out in the cytosol. They can multiply and releases antigens once they enter the cytosol (61). This process allows antigen processing and presentation through class I and II MHC molecules to stimulate effector responses from both CD4+ and CD8+ T cells. These features of Listeria can be leveraged for the production of tumor antigens by means of genetic engineering to potentiate the anti-tumor responses.
3.5 Bifidobacterium: Bifidobacterium is an anaerobic gram-positive bacterium that does not produce bacterial spores. Bifidobacteria are reported to have favorable effects on human health, which includes vitamin synthesis, pathogen defense, and the secretion of antimicrobial agents. In addition, it reduces the secretion of pro-inflammatory cytokines, enhances immune protective functions including activation of cytotoxic T cells and influences the interactions between natural killer (NK) cell and dendritic cells (62, 63). A number of reports have confirmed anti-tumor roles of Bifidobacteria (64)– (67). However, as opposed to other microbes like Clostridium spp., Bifidobacterium monotherapy did not show potent anti-tumor effects in preclinical studies in spite of successful tumor colonization (68). In contrast, these findings suggest that Bifidobacterium can be used as a delivery vehicle that can be easily genetically engineered to synthesize proteins of interest (69).
Bifidobacteria plays an important role in cancer prevention both in-vivo and in-vitro. Furthermore, some preclinical reports have shown its role in protecting DNA damage from carcinogens to inhibit the associated genotoxicities (70). Few other reports have also described a protective role of Bifidobacteria through immune surveillance and host’s immune system activation (71). Preclinical melanoma treatment study in mice have revealed that oral administration of Bifidobacterium provides an additive effect on tumor regression (72). Furthermore, tumor growth was significantly abolished when Bifidobacterium was used in combination with immune checkpoint inhibitors (72, 73). These anti-tumor effects were primarily manifested in the TME by increasing dendritic cell activation and CD8+ T-cell priming.
4. Classical cancer treatment regimen in conjunction with microbial therapy
Conventional cancer treatments include chemotherapy, radiotherapy, and immunotherapy. However, development of resistance to these therapies poses a major problem for improving overall survival of cancer patients. Both host and tumor secreted factors contributes in the development of resistance. As a result, there is an urgent need for the development of innovative therapies to combat the disease more effectively.
4.1 Radiotherapy
Radiation therapy has long been considered as one of the most effective cancer treatment. Radiotherapy can cause damage to both healthy and malignant cells but the primary goal of radiotherapy is to kill cancer cells while minimizing damage to normal cells. Radiotherapies are not very successful against solid tumors, as these tumors tend to have hypoxic regions that are resilient to radiation effects (74, 75). Use of anaerobic (obligate or facultative) bacteria can turn this limitation into an advantage against malignant cells. Bacterial therapy against solid tumors is an emerging area of investigation where lower and safer doses of ionizing radiations can be utilized to effectively kill cancer cells causing less to no harm to normal healthy cells. Importantly, bacteria-based anti-tumor therapies also alleviates the risks associated with gene therapies involving genetic alterations of cancer cells (74, 75). Human cancers
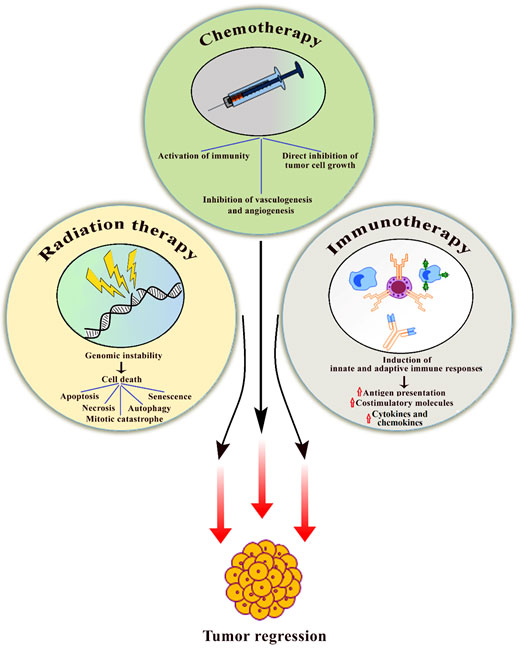
Figure 2. Schematic diagram highlights the use of bacteriotherapy in conjunction with classical cancer therapies and associated distinct mechanisms for targeting cancer cells. Over the last several years, bacteriotherapy in combination with traditional cancer therapies such as chemotherapy, radiotherapy, and immunotherapy has shown promising results. Bacteria inhibits tumor cell growth and induces apoptosis or senescence. Bacteria also helps in the activation of innate and adaptive immune system for the clearance of malignant cells.
|
have unique TME where tumor and stromal interactions play an important role in tumor growth and progression (76). TME is immune-dormant with altered metabolic state that allows the bacteria to colonize and thrive successfully within these tumors. Genetically engineered Salmonella holds the ability to selectively thrive and proliferate in the tumor microenvironment (33, 77). Salmonella can also express effector genes including the herpes simplex thymidine kinase (HSV-TK), which converts inactive GCV (ganciclovir) into its activated form leading to induction of anti-tumor activity (78). The combination of radio and bacteriotherapy has yielded much more than just additive anti-tumor effects. Attenuated S typhimurium strain ΔppGpp (guanosine 5’â€diphosphateâ€3’â€diphosphate) was genetically modified to encode a cytotoxic protein named as cytolysis A (ClyA) (79). ClyA is a poreâ€forming hemolytic protein that is normally absent in S typhimurium. The ClyA is cytotoxic to mammalian cells and induces caspase-mediated apoptosis. In addition, Clostridium novyi (C novyi)â€NT spores have been used in conjunction with radiotherapy to treat tumors (77). C novyiâ€NT spores alone had limited anti-tumor efficacy whereas in combination with radiotherapy yielded significant tumor regression. Combination therapies involving radiation and bacteria are significantly safer and can be successfully utilized to target selectively cancer cells without harming the adjacent normal healthy cells (Fig. 2). Certain probiotic bacteria, such as Lactobacilli and Bifidobacteria, have been shown to reduce the negative effects of radiotherapy (80). These probiotic bacteria have also been shown to aid in the recovery of damaged normal cells.
4.2 Chemotherapy
Chemotherapy still represents one of the mainstays of cancer treatment, in spite of causing systemic toxicity (81). Nowadays, chemotherapy-based treatments are gradually being set aside with the emergence of newer and effective treatment options like immunotherapy. The major limitation of chemotherapy is the development of drug-resistance, which occurs after the completion of the treatment course, when some of the residual cells acquire a higher invasive and metastatic potential, worsening the disease outcome. Henceforth, there is a greater need for the development of newer therapeutic targets and treatment strategies to combat the disease. Combination of chemo and bacteria-based therapy seems to be an attractive therapeutic strategy. Development of hypoxic regions is a hallmark feature of many human solid tumors, which are often resistant to anti-tumor therapies (74, 75). Importantly, bacteria not only holds the ability to thrive in such environment but also aid in specific killing of malignant cells.
Most commonly, these bacteria help in sensitizing the cancer cells, thereby increasing the therapeutic efficacy of chemotherapy drugs (74). In addition, these bacteria can also be used either directly or as vectors. Bacteria produces a number of toxins that show anti-tumor activity and mediate killing of cancer cells. Botulinum neurotoxin (BoNT) has been reported to target and lyse tumor cells directly; however, complete destruction of tumor cells has not been achieved (82). Interestingly, bacteria like S choleraesuis alone has demonstrated higher anti-cancer abilities and addition of chemotherapy drug cisplatin prolonged the development of tumor and improved overall survival (83). Therefore, addition of chemotherapy can compensate this insufficient lysis of target tumor cells and thereby, improve overall anti-tumor efficacy. These bacteria can also be used for developing bacterial cancer vaccines. With the advancement of genetic engineering, bacteria can be easily genetically engineered for specific targeting of cancer cells. Such genetically improved bacteria can specifically target cancer cells and can be used alone or in combination with canonical cancer treatment regimens (Fig. 2). Common side effects of chemotherapy are often associated with gastrointestinal tracts (84). Probiotic bacteria like lactobacillus plays an important role in culminating gastrointestinal side effects of chemotherapy (85). Use of probiotic bacteria during chemotherapy has resulted in fewer intestinal side effects in cancer patients.
4.3 Immunotherapy
Cancer cells avoid the immune mediated clearance through a variety of mechanisms including recruitment of immunosuppressive cells, secretion of cytokines/chemokines, expression of immune checkpoints, and downregulating major histocompatibility 1 (MHC1) surface presentation (86). Tumor-infiltrating immune cells in solid tumors are mainly derived from the innate immune system including macrophages. Tumor associated macrophages (TAMs) are the most common type of immune cell found in the tumor microenvironment (87). TAMs are known to promote tumor growth and metastasis (87). Macrophages are classified as M1 or M2, and TAMs are shown to have an M2-like phenotype. (87). Therefore, M2-like TAMs are considered as promising targets for cancer immunotherapy. Macrophages are highly plastic in nature and can rapidly change their phenotypes in response to their local signals in order to facilitate local tumor growth and distant metastasis (88). Cancer immunotherapy is based on boosting the host immune response that can specifically recognize and kill malignant cells. Cytotoxic T cells including CD8+ and CD4+ cells play predominant role in clearance of tumor cells. Moreover, combination of immune- and bacteriotherapy seems to be an attractive strategy for inhibiting tumor growth and progression (Fig. 2).
C novyi infection results in the production of various heat shock proteins including (Hsp70) (89). Hsp70 is mainly released from necrotic cells while bacteria release PAMPs (90). Hsp70 predominantly aids in the maturation of professional antigen presenting cells (APCs) such as dendritic cells (74, 91). These professional APCs are very important for the development of adaptive immunity, particularly cell-mediated immunity. PAMPs are unique molecules shared by various microbes that are required for bacterial survival but are absent in mammalian hosts. Bacterial lipopolysaccharide (LPS) and endotoxins are classical bacterial PAMPs. PAMPs elicit innate responses, which protect the host from infection. Inflammasomes are also activated by PAMPs, particularly NLRC4 inflammasomes (92). Inflammasomes are oligomer protein complexes that activates inflammatory signaling (93). Moreover, PAMPs are known to interact with tollâ€like receptors (TLRs) for inducing the secretion of pro-inflammatory cytokines including IL-12 (94). In addition, it enhances the expression of costimulatory molecules like CD40. Furthermore, these changes lead to the development of Th1 type immune response and increases the production of interferon gamma (IFNâ€γ) (95). Th1-type immune response includes the activation of tumor reactive cytolytic CD8+ effector T cells for tumor clearance (96). Bacteria like S typhimurium utilizes the typeâ€3 secretion system (T3SS) for infecting and penetrating melanoma cells while T3SS mutant strains lack the ability to penetrate host cells (97). S typhimurium does not completely kill infected target cells but their antigens are processed and presented over tumor cells that ultimately leads to their immune detection and clearance (97). Furthermore, bacterial compounds including CpG oligonucleotides can also activate APCs especially dendritic cells in melanoma, which further helps in tumor cell killing (98). E. coli strains are long been used for the production of recombinant proteins. They are also used for the delivery of tumor specific antigens in dendritic cells (99).
Cytokines and chemokines play important roles in tumor regression and immune activation. Several cytokines including IL-2, IL-15, IL-4, IFN-γ, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been delivered using genetically modified bacteria (9). Delivery of IL-2 through attenuated S. typhimurium has been progressed to human clinical trials (https://clinicaltrials.gov/ct2/show/NCT01099631). Furthermore, attenuated S. typhimurium injected in a mouse model promotes the secretion of flagellin B from another bacterium, Vibrio vulnificus in the tumor microenvironment that leads to macrophage polarization from M2 to M1 type (100). M2-type macrophages are pro-tumor and anti-inflammatory in nature as opposed to M1-type macrophages that are anti-tumor and pro-inflammatory. In addition, it promotes the production of anti-tumor cytokines including TNF-α and IL-1β. Another anti-cancer therapeutic approach to counter the immune suppressive mechanisms include the use of genetically engineered bacteria that make neutralizing nanobodies (nb) in the tumor microenvironment. These nb will help in boosting the overall anti-tumor immune response (101). For instance, E. coli Pir1+ was genetically modified to secrete CD47 nb that stimulates tumor-resident T cells and ultimately caused tumor regression (102).
5. Conclusion
Canonical anti-cancer therapies face significant challenges because of the uncanny behavior of cancer cells. There are both benefits and drawbacks in using bacteria therapeutically for cancer therapy. Although conventional cancer therapies remain the mainstay treatment, microbial therapy has produced impressive results due to high specificity, manageable post administration, and anti-cancer effects. However, many challenges still prevail in using microbes as anti-tumor agents, which primarily includes toxicities associated with bacterial administration, DNA instability, modest efficiency, and selection of safer and effective bacterial strain. In the near future, genetically modified bacteria may overcome these limitations, making its therapeutic use for targeted anti-tumor therapies more feasible.
References:
|
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 May;71(3):209-249.
https://doi.org/10.3322/caac.21660 PMid:33538338
|
|
2. Shiels MS, Gibson T, Sampson J, Albanes D, Andreotti G, Beane Freeman L, et al. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. J Clin Oncol. 2014 Dec 10;32(35):3989-3995.
https://doi.org/10.1200/JCO.2014.56.8220 PMid:25385740 PMCid:PMC4251962
|
|
|
3. Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002 Apr;55(4):244-265. https://doi.org/10.1136/jcp.55.4.244 PMid:11919208 PMCid:PMC1769629
|
|
|
4. Liu X, Chen Y, Wang Y, Dong X, Wang J, Tang J, et al. Cancer risk in patients with hepatitis C virus infection: a population-based study in Sweden. Cancer Med. 2017 May;6(5):1135-1140. https://doi.org/10.1002/cam4.988 PMid:28374973 PMCid:PMC5527979
|
|
|
5. Mahale P, Engels EA, Koshiol J. Hepatitis B virus infection and the risk of cancer in the elderly US population. Int J Cancer. 2019 Feb 1;144(3):431-439.
https://doi.org/10.1002/ijc.31643 PMid:29974477
|
|
|
6. Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013 Sep 19;501(7467):338-345. https://doi.org/10.1038/nature12625 PMid:24048066
|
|
|
7. Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. 2014 Jun 2;7:9-18. https://doi.org/10.4137/CGM.S11285 PMid:24926201 PMCid:PMC4051818
|
|
|
8. Seager RJ, Hajal C, Spill F, Kamm RD, Zaman MH. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg Sci Phys Oncol. 2017 Jul 28;3. https://doi.org/10.1088/2057-1739/aa7e86 PMid:30079253 PMCid:PMC6070160
|
|
|
9. Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol. 2019 Feb;54(2):407-419.
|
|
|
10. Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18(12):727-743.
https://doi.org/10.1038/s41568-018-0070-z PMid:30405213 PMCid:PMC6902869
|
|
|
11. Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010 Nov;10(11):785-794. https://doi.org/10.1038/nrc2934
PMid:20944664 PMCid:PMC3756932
|
|
|
12. Van Mellaert L, Barbé S, Anné J. Clostridium spores as anti-tumour agents. Trends Microbiol. 2006 Apr;14(4):190-196.
https://doi.org/10.1016/j.tim.2006.02.002 PMid:16500103
|
|
|
13. Hoffman RM. Tumor-seeking Salmonella amino acid auxotrophs. Curr Opin Biotechnol. 2011 Dec;22(6):917-923. https://doi.org/10.1016/j.copbio.2011.03.009
PMid:21498066
|
|
|
14. Lamm DL. BCG immunotherapy for transitional-cell carcinoma in situ of the bladder. Oncology. 1995 Oct;9(10):947-52, 955, discussion 955.
|
|
|
15. Sharma P, Old LJ, Allison JP. Immunotherapeutic strategies for high-risk bladder cancer. Semin Oncol. 2007 Apr;34(2):165-172.
https://doi.org/10.1053/j.seminoncol.2006.12.004 PMid:17382800 PMCid:PMC6009830
|
|
|
16. Yamada T, Goto M, Punj V, Zaborina O, Chen ML, Kimbara K, et al. Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci USA. 2002 Oct 29;99(22):14098-14103. https://doi.org/10.1073/pnas.222539699 PMid:12393814 PMCid:PMC137843
|
|
|
17. Taranta M, Bizzarri AR, Cannistraro S. Probing the interaction between p53 and the bacterial protein azurin by single molecule force spectroscopy. J Mol Recognit. 2008 Feb;21(1):63-70. https://doi.org/10.1002/jmr.869 PMid:18247358
|
|
|
18. Charan M, Dravid P, Cam M, Audino A, Gross AC, Arnold MA, et al. GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevent primary tumor growth and metastasis in Ewing sarcoma. Int J Cancer. 2020 Jun 1;146(11):3184-3195. https://doi.org/10.1002/ijc.32743
PMid:31621900 PMCid:PMC7440656
|
|
|
19. Cam M, Charan M, Welker AM, Dravid P, Studebaker AW, Leonard JR, et al. ΔNp73/ETS2 complex drives glioblastoma pathogenesis- targeting downstream mediators by rebastinib prolongs survival in preclinical models of glioblastoma. Neuro Oncol. 2020 Mar 5;22(3):345-356. https://doi.org/10.1093/neuonc/noz190
PMid:31763674 PMCid:PMC7058445
|
|
|
20. Chen L, He Z, Qin L, Li Q, Shi X, Zhao S, et al. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS One. 2011 Sep 9;6(9):e24407. https://doi.org/10.1371/journal.pone.0024407 PMid:21931708 PMCid:PMC3170332
|
|
|
21. Wang B, Li Q, Wang J, Zhao S, Nashun B, Qin L, et al. Plasmodium infection inhibits tumor angiogenesis through effects on tumor-associated macrophages in a murine implanted hepatoma model. Cell Commun Signal. 2020 Sep 24;18(1):157. https://doi.org/10.1186/s12964-020-00570-5 PMid:32972437 PMCid:PMC7513281
|
|
|
22. Pan J, Ma M, Qin L, Kang Z, Adah D, Tao Z, et al. Plasmodium infection inhibits triple negative 4T1 breast cancer potentially through induction of CD8+ T cell-mediated antitumor responses in mice. Biomed Pharmacother. 2021 Mar 3;138:111406. https://doi.org/10.1016/j.biopha.2021.111406 PMid:33676307
|
|
|
23. Tanveer A, Allen SM, Jackson KE, Charan M, Ralph SA, Habib S. An FtsH protease is recruited to the mitochondrion of Plasmodium falciparum. PLoS One. 2013 Sep 13;8(9):e74408.https://doi.org/10.1371/journal.pone.0074408 PMid:24058559 PMCid:PMC3772908
|
|
|
24. Charan M, Singh N, Kumar B, Srivastava K, Siddiqi MI, Habib S. Sulfur mobilization for Fe-S cluster assembly by the essential SUF pathway in the Plasmodium falciparum apicoplast and its inhibition. Antimicrob Agents Chemother. 2014 Jun;58(6):3389-3398. https://doi.org/10.1128/AAC.02711-13
PMid:24709262 PMCid:PMC4068488
|
|
|
25. Kumar B, Chaubey S, Shah P, Tanveer A, Charan M, Siddiqi MI, et al. Interaction between sulphur mobilisation proteins SufB and SufC: evidence for an iron-sulphur cluster biogenesis pathway in the apicoplast of Plasmodium falciparum. Int J Parasitol. 2011 Aug 1;41(9):991-999.
https://doi.org/10.1016/j.ijpara.2011.05.006 PMid:21722645
|
|
|
26. Ellis T, Eze E, Raimi-Abraham BT. Malaria and Cancer: a critical review on the established associations and new perspectives. Infect Agents Cancer. 2021 May 13;16(1):33. https://doi.org/10.1186/s13027-021-00370-7 PMid:33985540 PMCid:PMC8117320
|
|
|
27. Yang Y, Liu Q, Lu J, Adah D, Yu S, Zhao S, et al. Exosomes from Plasmodium-infected hosts inhibit tumor angiogenesis in a murine Lewis lung cancer model. Oncogenesis. 2017 Jun 26;6(6):e351.https://doi.org/10.1038/oncsis.2017.52 PMid:28650446 PMCid:PMC5519199
|
|
|
28. Liu Q, Yang Y, Tan X, Tao Z, Adah D, Yu S, et al. Plasmodium parasite as an effective hepatocellular carcinoma antigen glypican-3 delivery vector. Oncotarget. 2017 Apr 11;8(15):24785-24796. https://doi.org/10.18632/oncotarget.15806 PMid:28445973 PMCid:PMC5421888
|
|
|
29. Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment. Anticancer Drugs. 2016 Apr;27(4):269-277. https://doi.org/10.1097/CAD.0000000000000337 PMid:26813865 PMCid:PMC4777220
|
|
|
30. Hoffman RM, editor. Bacterial therapy of cancer. New York, NY: Springer New York; 2016.
|
|
|
31. McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154-158.
|
|
|
32. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976 Aug;116(2):180-183.
https://doi.org/10.1016/S0022-5347(17)58737-6
|
|
|
33. Guallar-Garrido S, Julián E. Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. Immunotargets Ther. 2020 Feb 13;9:1-11.
https://doi.org/10.2147/ITT.S202006 PMid:32104666 PMCid:PMC7025668
|
|
|
34. Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci USA. 2001 Dec 18;98(26):15155-15160. https://doi.org/10.1073/pnas.251543698 PMid:11724950 PMCid:PMC64999
|
|
|
35. Cheadle EJ, Jackson AM. Bugs as drugs for cancer. Immunology. 2002 Sep;107(1):10-19. https://doi.org/10.1046/j.1365-2567.2002.01498.x PMid:12225358 PMCid:PMC1782780
|
|
|
36. Adkins I, Holubova J, Kosova M, Sadilkova L. Bacteria and their toxins tamed for immunotherapy. Curr Pharm Biotechnol. 2012 Jun;13(8):1446-1473.
https://doi.org/10.2174/138920112800784835 PMid:22339216
|
|
|
37. Kim J-E, Phan TX, Nguyen VH, Dinh-Vu H-V, Zheng JH, Yun M, et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics. 2015 Oct 6;5(12):1328-1342. https://doi.org/10.7150/thno.11432 PMid:26516371 PMCid:PMC4615736
|
|
|
38. Phan TX, Nguyen VH, Duong MT-Q, Hong Y, Choy HE, Min J-J. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol. 2015 Nov;59(11):664-675. https://doi.org/10.1111/1348-0421.12333 PMid:26500022
|
|
|
39. Park S-H, Zheng JH, Nguyen VH, Jiang S-N, Kim D-Y, Szardenings M, et al. RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy. Theranostics. 2016 Jun 20;6(10):1672-1682. https://doi.org/10.7150/thno.16135
PMid:27446500 PMCid:PMC4955065
|
|
|
40. Massa PE, Paniccia A, Monegal A, de Marco A, Rescigno M. Salmonella engineered to express CD20-targeting antibodies and a drug-converting enzyme can eradicate human lymphomas. Blood. 2013 Aug 1;122(5):705-714. https://doi.org/10.1182/blood-2012-12-474098 PMid:23736700
|
|
|
41. Jain RK, Forbes NS. Can engineered bacteria help control cancer? Proc Natl Acad Sci USA. 2001 Dec 18;98(26):14748-14750.
https://doi.org/10.1073/pnas.261606598 PMid:11752416 PMCid:PMC64926
|
|
|
42. Green DB, Kawashima A, Menias CO, Tanaka T, Redelman-Sidi G, Bhalla S, et al. Complications of intravesical BCG immunotherapy for bladder cancer. Radiographics. 2019 Feb;39(1):80-94. https://doi.org/10.1148/rg.2019180014 PMid:30526332
|
|
|
43. Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol. 2008 Jan;179(1):53-56.
https://doi.org/10.1016/j.juro.2007.08.122 PMid:17997439
|
|
|
44. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. 1976. J Urol. 2002 Feb;167(2 Pt 2):891-3; discussion 893. https://doi.org/10.1016/S0022-5347(02)80294-4
|
|
|
45. Nanney LB, Wamil BD, Whitsitt J, Cardwell NL, Davidson JM, Yan HP, et al. CM101 stimulates cutaneous wound healing through an anti-angiogenic mechanism. Angiogenesis. 2001;4(1):61-70. https://doi.org/10.1023/A:1016752925761 PMid:11824380
|
|
|
46. Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529-564.
https://doi.org/10.1016/0163-7258(94)90023-X
|
|
|
47. Marzhoseyni Z, Shojaie L, Tabatabaei SA, Movahedpour A, Safari M, Esmaeili D, et al. Streptococcal bacterial components in cancer therapy. Cancer Gene Ther. 2021 Mar 22; https://doi.org/10.1038/s41417-021-00308-6 PMid:33753868
|
|
|
48. Felgner S, Kocijancic D, Frahm M, Weiss S. Bacteria in cancer therapy: renaissance of an old concept. Int J Microbiol. 2016 Mar 8;2016:8451728.
https://doi.org/10.1155/2016/8451728 PMid:27051423 PMCid:PMC4802035
|
|
|
49. Paton AW, Morona R, Paton JC. Bioengineered microbes in disease therapy. Trends Mol Med. 2012 Jul;18(7):417-425.
https://doi.org/10.1016/j.molmed.2012.05.006 PMid:22721939
|
|
|
50. Liu S, Xu X, Zeng X, Li L, Chen Q, Li J. Tumor-targeting bacterial therapy: A potential treatment for oral cancer (Review). Oncol Lett. 2014 Dec;8(6):2359-2366. https://doi.org/10.3892/ol.2014.2525 PMid:25364397 PMCid:PMC4214492
|
|
|
51. Staedtke V, Roberts NJ, Bai R-Y, Zhou S. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016 Jun;3(2):144-152.
https://doi.org/10.1016/j.gendis.2016.01.003 PMid:30258882 PMCid:PMC6150096
|
|
|
52. Roberts NJ, Zhang L, Janku F, Collins A, Bai R-Y, Staedtke V, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014 Aug 13;6(249):249ra111. https://doi.org/10.1126/scitranslmed.3008982 PMid:25122639 PMCid:PMC4399712
|
|
|
53. Fabricius EM, Schneeweiss U, Schau HP, Schmidt W, Benedix A. Quantitative investigations into the elimination of in vitro-obtained spores of the non-pathogenic Clostridium butyricum strain CNRZ 528, and their persistence in organs of different species following intravenous spore administration. Res Microbiol. 1993 Dec;144(9):741-753. https://doi.org/10.1016/0923-2508(93)90038-4
|
|
|
54. Rhim T, Lee DY, Lee M. Hypoxia as a target for tissue specific gene therapy. J Control Release. 2013 Dec 10;172(2):484-494.
https://doi.org/10.1016/j.jconrel.2013.05.021 PMid:23742881
|
|
|
55. Barbé S, Van Mellaert L, Theys J, Geukens N, Lammertyn E, Lambin P, et al. Secretory production of biologically active rat interleukin-2 by Clostridium acetobutylicum DSM792 as a tool for anti-tumor treatment. FEMS Microbiol Lett. 2005 May 1;246(1):67-73. https://doi.org/10.1016/j.femsle.2005.03.037
PMid:15869963
|
|
|
56. Zhang YL, Lü R, Chang ZS, Zhang WQ, Wang QB, Ding SY, et al. Clostridium sporogenes delivers interleukin-12 to hypoxic tumours, producing antitumour activity without significant toxicity. Lett Appl Microbiol. 2014 Dec;59(6):580-586. https://doi.org/10.1111/lam.12322 PMid:25163827
|
|
|
57. Nuyts S, Van Mellaert L, Theys J, Landuyt W, Bosmans E, Anné J, et al. Radio-responsive recA promoter significantly increases TNFalpha production in recombinant clostridia after 2 Gy irradiation. Gene Ther. 2001 Aug;8(15):1197-1201. https://doi.org/10.1038/sj.gt.3301499 PMid:11509951
|
|
|
58. Groot AJ, Mengesha A, van der Wall E, van Diest PJ, Theys J, Vooijs M. Functional antibodies produced by oncolytic clostridia. Biochem Biophys Res Commun. 2007 Dec 28;364(4):985-989. https://doi.org/10.1016/j.bbrc.2007.10.126 PMid:17971292
|
|
|
59. Chen G-W, Wu M, Liu W-K, Xie M-M, Zhang W-S, Fan E-G, et al. Reactive oxygen species inhibits Listeria monocytogenes invasion into HepG2 epithelial cells. Food Sci Nutr. 2018 Sep;6(6):1501-1507. https://doi.org/10.1002/fsn3.615 PMid:30258592 PMCid:PMC6145247
|
|
|
60. Deng W, Lira V, Hudson TE, Lemmens EE, Hanson WG, Flores R, et al. Recombinant Listeria promotes tumor rejection by CD8+ T cell-dependent remodeling of the tumor microenvironment. Proc Natl Acad Sci USA. 2018 Aug 7;115(32):8179-8184. https://doi.org/10.1073/pnas.1801910115 PMid:30038013 PMCid:PMC6094133
|
|
|
61. Dussurget O, Bierne H, Cossart P. The bacterial pathogen Listeria monocytogenes and the interferon family: type I, type II and type III interferons. Front Cell Infect Microbiol. 2014 Apr 28;4:50. https://doi.org/10.3389/fcimb.2014.00050 PMid:24809023 PMCid:PMC4009421
|
|
|
62. Ruan Y, Rezelj S, Bedina Zavec A, Anderluh G, Scheuring S. Listeriolysin O Membrane Damaging Activity Involves Arc Formation and Lineaction -- Implication for Listeria monocytogenes Escape from Phagocytic Vacuole. PLoS Pathog. 2016 Apr 22;12(4):e1005597.
https://doi.org/10.1371/journal.ppat.1005597 PMid:27104344 PMCid:PMC4841516
|
|
|
63. Ambalam P, Raman M, Purama RK, Doble M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol. 2016 Feb 19;30(1):119-131. https://doi.org/10.1016/j.bpg.2016.02.009 PMid:27048903
|
|
|
64. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: A summary of the evidence. Am Fam Physician. 2017 Aug 1;96(3):170-178.
|
|
|
65. Liboredo JC, Anastácio LR, Pelúzio M do CG, Valente FX, Penido LCP, Nicoli JR, et al. Effect of probiotics on the development of dimethylhydrazine-induced preneoplastic lesions in the mice colon. Acta Cir Bras. 2013 May;28(5):367-372. https://doi.org/10.1590/S0102-86502013000500008 PMid:23702939
|
|
|
66. Wei C, Xun AY, Wei XX, Yao J, Wang JY, Shi RY, et al. Bifidobacteria Expressing Tumstatin Protein for Antitumor Therapy in Tumor-Bearing Mice. Technol Cancer Res Treat. 2016 Jun;15(3):498-508. https://doi.org/10.1177/1533034615581977 PMid:25969440
|
|
|
67. Kim K-A, Jung I-H, Park S-H, Ahn Y-T, Huh C-S, Kim D-H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One. 2013 Apr 29;8(4):e62409. https://doi.org/10.1371/journal.pone.0062409 PMid:23638073 PMCid:PMC3639287
|
|
|
68. Lai L-R, Hsieh S-C, Huang H-Y, Chou C-C. Effect of lactic fermentation on the total phenolic, saponin and phytic acid contents as well as anti-colon cancer cell proliferation activity of soymilk. J Biosci Bioeng. 2013 May;115(5):552-556. https://doi.org/10.1016/j.jbiosc.2012.11.022 PMid:23290992
|
|
|
69. Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003 Sep;4(9):548-556.
https://doi.org/10.1016/S1470-2045(03)01194-X
|
|
|
70. Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Bifidobacterium longum as a delivery system for cancer gene therapy: selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000 Feb;7(2):269-274. https://doi.org/10.1038/sj.cgt.7700122 PMid:10770636
|
|
|
71. Pool-Zobel BL, Neudecker C, Domizlaff I, Ji S, Schillinger U, Rumney C, et al. Lactobacillus- and bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer. 1996;26(3):365-380. https://doi.org/10.1080/01635589609514492 PMid:8910918
|
|
|
72. Yasui H, Ohwaki M. Enhancement of immune response in Peyer's patch cells cultured with Bifidobacterium breve. J Dairy Sci. 1991 Apr;74(4):1187-1195.
https://doi.org/10.3168/jds.S0022-0302(91)78272-6
|
|
|
73. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015 Nov 27;350(6264):1084-1089.https://doi.org/10.1126/science.aac4255 PMid:26541606 PMCid:PMC4873287
|
|
|
74. Wang F. Bifidobacterium improves the outcome of immune checkpoint blockade by modulating Treg cell function. The Journal of Immunology. 2020;204(1 Supplement):90.2-90.2.
|
|
|
75. Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M, et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019 Apr 5;8(6):3167-3181. https://doi.org/10.1002/cam4.2148 PMid:30950210 PMCid:PMC6558487
|
|
|
76. Sieow BF-L, Wun KS, Yong WP, Hwang IY, Chang MW. Tweak to treat: reprograming bacteria for cancer treatment. Trends Cancer. 2020 Dec 7;
https://doi.org/10.1016/j.trecan.2020.11.004 PMid:33303401
|
|
|
77. Charan M, Verma AK, Hussain S, Misri S, Mishra S, Majumder S, et al. Molecular and Cellular Factors Associated with Racial Disparity in Breast Cancer. Int J Mol Sci. 2020 Aug 18;21(16). https://doi.org/10.3390/ijms21165936 PMid:32824813 PMCid:PMC7460595
|
|
|
78. Liu X, Jiang S, Piao L, Yuan F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp Anim. 2016 Nov 1;65(4):413-418. https://doi.org/10.1538/expanim.16-0033 PMid:27301721 PMCid:PMC5111844
|
|
|
79. Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997 Oct 15;57(20):4537-4544.
|
|
|
80. Hunt S, Green J, Artymiuk PJ. Hemolysin E (HlyE, ClyA, SheA) and related toxins. Adv Exp Med Biol. 2010;677:116-126.
https://doi.org/10.1007/978-1-4419-6327-7_10 PMid:20687485
|
|
|
81. Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 2010 May 5;5:31.
https://doi.org/10.1186/1748-717X-5-31 PMid:20444243 PMCid:PMC2874795
|
|
|
82. Patel M, Eckburg A, Gantiwala S, Hart Z, Dein J, Lam K, et al. Resistance to molecularly targeted therapies in melanoma. Cancers (Basel). 2021 Mar 5;13(5).
https://doi.org/10.3390/cancers13051115 PMid:33807778 PMCid:PMC7961479
|
|
|
83. Ansiaux R, Gallez B. Use of botulinum toxins in cancer therapy. Expert Opin Investig Drugs. 2007 Feb;16(2):209-218.
https://doi.org/10.1517/13543784.16.2.209 PMid:17243940
|
|
|
84. Lee C-H, Wu C-L, Tai Y-S, Shiau A-L. Systemic administration of attenuated Salmonella choleraesuis in combination with cisplatin for cancer therapy. Mol Ther. 2005 May;11(5):707-716. https://doi.org/10.1016/j.ymthe.2005.01.008 PMid:15851009
|
|
|
85. Boussios S, Pentheoudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol. 2012;25(2):106-118.
|
|
|
86. Lu K, Dong S, Wu X, Jin R, Chen H. Probiotics in Cancer. Front Oncol. 2021 Mar 12;11:638148. https://doi.org/10.3389/fonc.2021.638148
PMid:33791223 PMCid:PMC8006328
|
|
|
87. Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015 Dec;35 Suppl:S185-S198. https://doi.org/10.1016/j.semcancer.2015.03.004 PMid:25818339
|
|
|
88. Ge Z, Ding S. The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Front Oncol. 2020 Nov 3;10:590941. https://doi.org/10.3389/fonc.2020.590941 PMid:33224886 PMCid:PMC7670061
|
|
|
89. Grebić D, Gulić T, StarÄević A, Alvirović M, Blagojević Zagorac G, Valković Zujić P, et al. The role of innate immunity in the pathogenesis of breast cancer. Breast Care (Basel). 2021 Feb;16(1):1-5. https://doi.org/10.1159/000507314 PMid:33716626
|
|
|
90. Gelman AE, Turka LA. Autoimmunity heats up. Nat Med. 2003 Dec;9(12):1465-1466. https://doi.org/10.1038/nm1203-1465 PMid:14647523
|
|
|
91. Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med. 2003 Dec;9(12):1469-1476. https://doi.org/10.1038/nm962 PMid:14625545
|
|
|
92. Kettern N, Rogon C, Limmer A, Schild H, Höhfeld J. The Hsc/Hsp70 co-chaperone network controls antigen aggregation and presentation during maturation of professional antigen presenting cells. PLoS One. 2011 Jan 20;6(1):e16398. https://doi.org/10.1371/journal.pone.0016398 PMid:21283720 PMCid:PMC3024426
|
|
|
93. Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020 Jun 9;6:36.
https://doi.org/10.1038/s41421-020-0167-x PMid:32550001 PMCid:PMC7280307
|
|
|
94. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016 Jun 13;16(7):407-420.
https://doi.org/10.1038/nri.2016.58 PMid:27291964
|
|
|
95. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009 Apr;22(2):240-73, Table of Contents.
https://doi.org/10.1128/CMR.00046-08 PMid:19366914 PMCid:PMC2668232
|
|
|
96. Berger A. Th1 and Th2 responses: what are they? BMJ. 2000 Aug 12;321(7258):424. https://doi.org/10.1136/bmj.321.7258.424 PMid:10938051 PMCid:PMC27457
|
|
|
97. Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003 Feb 3;197(3):387-393. https://doi.org/10.1084/jem.20021683 PMid:12566422 PMCid:PMC2193835
|
|
|
98. Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005 May 1;65(9):3920-3927. https://doi.org/10.1158/0008-5472.CAN-04-3002 PMid:15867392
|
|
|
99. Lange MJ, Burke DH, Chaput JC. Activation of innate immune responses by a cpg oligonucleotide sequence composed entirely of threose nucleic acid. Nucleic Acid Ther. 2019;29(1):51-59. https://doi.org/10.1089/nat.2018.0751 PMid:30526333 PMCid:PMC6939586
|
|
|
100. Rescigno M, Valzasina B, Bonasio R, Urbano M, Ricciardi-Castagnoli P. Dendritic cells, loaded with recombinant bacteria expressing tumor antigens, induce a protective tumor-specific response. Clin Cancer Res. 2001 Mar;7(3 Suppl):865s-870s.
|
|
|
101. Zheng JH, Nguyen VH, Jiang S-N, Park S-H, Tan W, Hong SH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017 Feb 8;9(376). https://doi.org/10.1126/scitranslmed.aak9537 PMid:28179508
|
|
|
102. Blanchetot C, Verzijl D, Mujić-Delić A, Bosch L, Rem L, Leurs R, et al. Neutralizing nanobodies targeting diverse chemokines effectively inhibit chemokine function. J Biol Chem. 2013 Aug 30;288(35):25173-25182. https://doi.org/10.1074/jbc.M113.467969 PMid:23836909 PMCid:PMC3757181
|
|
|
103. Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 2019 Jul 3;25(7):1057-1063. https://doi.org/10.1038/s41591-019-0498-z PMid:31270504 PMCid:PMC6688650
|
|
|
104. Mizrahi A, Czerniak A, Levy T, Amiur S, Gallula J, Matouk I, et al. Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med. 2009 Aug 6;7:69. https://doi.org/10.1186/1479-5876-7-69 PMid:19656414 PMCid:PMC2734756
|
|
|
105. Scaiewicz V, Sorin V, Fellig Y, Birman T, Mizrahi A, Galula J, et al. Use of H19 Gene Regulatory Sequences in DNA-Based Therapy for Pancreatic Cancer. J Oncol. 2010 Oct 28;2010:178174. https://doi.org/10.1155/2010/178174 PMid:21052499 PMCid:PMC2967839
|
|
|
106. Hasenpusch G, Pfeifer C, Aneja MK, Wagner K, Reinhardt D, Gilon M, et al. Aerosolized BC-819 inhibits primary but not secondary lung cancer growth. PLoS One. 2011 Jun 8;6(6):e20760. https://doi.org/10.1371/journal.pone.0020760 PMid:21687669 PMCid:PMC3110766
|
|
|
107. Yang WS, Park S-O, Yoon A-R, Yoo JY, Kim MK, Yun C-O, et al. Suicide cancer gene therapy using pore-forming toxin, streptolysin O. Mol Cancer Ther. 2006 Jun;5(6):1610-1619. https://doi.org/10.1158/1535-7163.MCT-05-0515 PMid:16818521
|
|
|
108. Fotoohi-Ardakani G, Kheirollahi M, Zarei Jaliani H, Noorian M, Ansariniyia H. Targeting MCF-7 Cell Line by Listeriolysin O Pore Forming Toxin Fusion with AHNP Targeted Peptide. Adv Biomed Res. 2019 May 27;8:33. https://doi.org/10.4103/abr.abr_18_19 PMid:31259162 PMCid:PMC6543864
|
|
|
109. Ishii KJ, Kawakami K, Gursel I, Conover J, Joshi BH, Klinman DM, et al. Antitumor therapy with bacterial DNA and toxin: complete regression of established tumor induced by liposomal CpG oligodeoxynucleotides plus interleukin-13 cytotoxin. Clin Cancer Res. 2003 Dec 15;9(17):6516-6522.
|
|
|
110. Fiedler T, Strauss M, Hering S, Redanz U, William D, Rosche Y, et al. Arginine deprivation by arginine deiminase of Streptococcus pyogenes controls primary glioblastoma growth in vitro and in vivo. Cancer Biol Ther. 2015 Mar 16;16(7):1047-1055. https://doi.org/10.1080/15384047.2015.1026478 PMid:25774632 PMCid:PMC4623118
|
|
|
111. Cheung CY, Holzwarth MA. Fetal adrenal VIP: distribution and effect on medullary catecholamine secretion. Peptides. 1986 Jun;7(3):413-418.
https://doi.org/10.1016/0196-9781(86)90007-0
|
|
|
112. Ahmadi S, Ghollasi M, Hosseini HM. The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb Pathog. 2017 Oct;111:193-197.
https://doi.org/10.1016/j.micpath.2017.08.037 PMid:28867631
|
|
|
113. Arunmanee W, Ecoy GAU, Khine HEE, Duangkaew M, Prompetchara E, Chanvorachote P, et al. Colicin N Mediates Apoptosis and Suppresses Integrin-Modulated Survival in Human Lung Cancer Cells. Molecules. 2020 Feb 13;25(4). https://doi.org/10.3390/molecules25040816 PMid:32069989 PMCid:PMC7070259
|
|
|
114. Paiva AD, de Oliveira MD, de Paula SO, Baracat-Pereira MC, Breukink E, Mantovani HC. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology (Reading, Engl). 2012 Nov;158(Pt 11):2851-2858. https://doi.org/10.1099/mic.0.062190-0 PMid:22956757
|
|
|
115. Park SY, Kim J-H, Lee YJ, Lee SJ, Kim Y. Surfactin suppresses TPA-induced breast cancer cell invasion through the inhibition of MMP-9 expression. Int J Oncol. 2013 Jan;42(1):287-296. https://doi.org/10.3892/ijo.2012.1695 PMid:23151889
|
|
|