Abstract
Extensive anatomical and physiological data from thalamus have linked terminal morphology to function. In thalamus, these are grounds for a theorized functional dichotomy between large terminals that transmit receptive fields and small terminals that play a mere modulatory role, and this framework has helped characterize thalamic function, particularly in nuclei which relay information from cortical areas. However, recent work by myself and my colleagues demonstrates that populations of terminals in thalamus exhibit more nuanced variation in terminal size. This review examines evidence for dichotomous terminal populations in thalamus, our new dataset challenging this precedent, and its implications to be addressed in future experiments.
Keywords forebrain circuitry, cortical laminae, neuronal properties, structure and function
Introduction
The thalamus is a centrally located brain region that relays information to and from cortex, constituting diverse brain circuits. Nearly all sensory information ascends through thalamus before reaching cortex. In addition, cortical regions also communicate with each other via pathways traversing the thalamus. As such, the thalamus is divided into functionally discrete nuclei serving distinct sensory, sensorimotor, or cognitive modalities. Despite this functional diversity, neurons in thalamic nuclei, particularly sensory nuclei, exhibit a stereotyped pattern of connectivity. Namely, a minority of afferents, known as drivers, strongly impact thalamic relay cells and dictate their receptive fields (i.e., are the source of information to be relayed), while another class of inputs, known as modulators, yield a more modest impact and may serve to adjust the information coming from drivers (1, 2).
Morphological and physiological data have established structural and functional features that classify synapses in thalamus as driver or modulator. At the morphological level, driver terminals generally arise from thick axons, are larger, and contact proximal dendrites on their target cell. In contrast, modulators form smaller terminals that protrude from thinner axons and typically contact distal dendrites (3). Physiologically, drivers elicit large, depressing postsynaptic potentials (subsequent potentials are smaller in size compared to the first) that are mediated by ionotropic receptors and are likely to drive an action potential in their target cells. Modulators, on the other hand, elicit weaker but facilitating postsynaptic potentials (potentials increase in size following the first) that also activate metabotropic receptors in their target cells (3) (Figure 1A).
From the rich literature describing the structure-function relationship of drivers and modulators, we first illustrate this dichotomy using evidence from two visual nuclei. In the dorsal lateral geniculate nucleus (LGN), where this relationship was first established, we present the evidence that distinguished retinal drivers from modulators arising from layer 6 of cortex (L6). Next, we present a parallel dichotomy in the pulvinar, where drivers are instead derived from layer 5 (L5) of cortex.
Recently, we used bulk-labelling of L5 cortical neurons to characterize the properties of their terminals throughout thalamus (4). Where it has been measured physiologically, L5 exclusively exhibits a driver profile; however, our anatomical data revealed structural differences in driver terminals across thalamic nuclei, suggesting potential functional variability within these projections. Such a variable profile for L5 terminals suggests subtle diversity within the driver/modulator framework that may be overlooked by dichotomizing these projection types. Here, we first present the classical framework before discussing the implications of potential intermediate projection subtypes from cortical L5.
Drivers and modulators in the LGN (Figure 1A)
A subset of thalamic nuclei, termed first-order, inherit their driver inputs from subcortical sources, such as primary sensory structures. In the visual system, retinal afferents drive relay cells in the first-order nucleus LGN, dictating their receptive fields. This is evidenced by in vivo electrophysiological recordings of retinal ganglion and LGN cell pairs, which demonstrate short latency (~4.5ms) spike transmission from retina to LGN, indicative of a monosynaptic connection, and shared receptive field properties (5). Further, tetrodotoxin injected into the eye eliminated spikes locally among retinal ganglion cells, but also downstream in LGN, conclusively demonstrating LGN’s dependence on information arriving via this synapse (6).
Parallel experiments have identified another set of inputs to LGN, which appear to lack potency relative to those from the retina. These are from the primary visual cortex, where LGN-bound projections arise exclusively in layer 6 (L6) (2). These L6 afferents do not transmit receptive field properties to LGN. While cross-correlograms indicate monosynaptic connections from L6 to LGN 7, inactivation reduces but does not abolish LGN activity8. Compared to the overall reduction in LGN activity at a population level, more targeted studies have shown the effects of L6 on the target LGN cell vary according to how well-matched the two are in receptive field. Paired recordings with focal cortical activation of L6 show that this input increases activity for LGN cells with overlapping receptive fields, but decreases activity for LGN cells with orthogonal receptive fields(9, 10). These experiments suggest that modulators may play a role in gain function 11.
As described above, driver inputs appear to more potently impact targets compared to modulators in vivo, and these different potencies are also observed in vitro, as differences in their synaptic properties. Recording intracellularly in LGN in vitro, McCormick and von Krosigk observed distinct synaptic properties of cortical and retinal afferents. Electrical stimulation of the optic tract elicited large fast potentials that depressed with repeated stimulation, and of the corticothalamic tract elicited smaller, facilitating fast potentials and an additional long-lasting potential mediated by mGluRs (12).
Anatomical analysis of retinal and cortical inputs has identified features that reflect their relative impacts. Initially, transection of the optic nerve or removal of striate cortex were used to
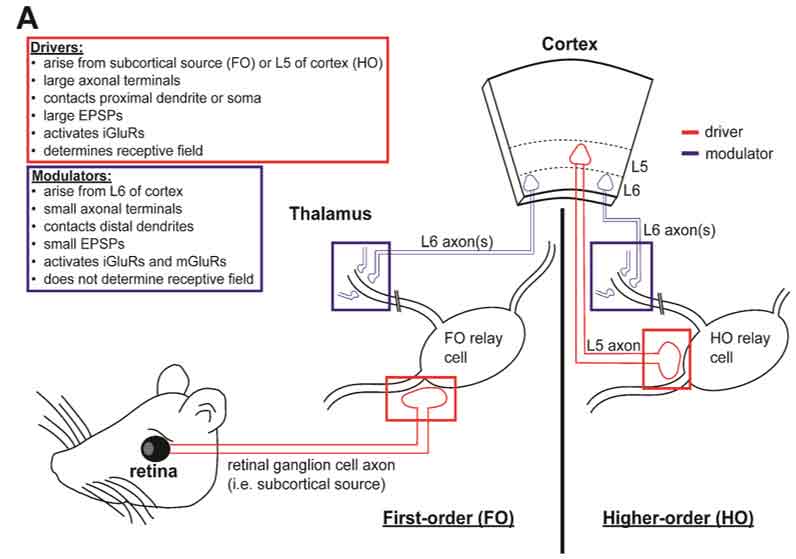
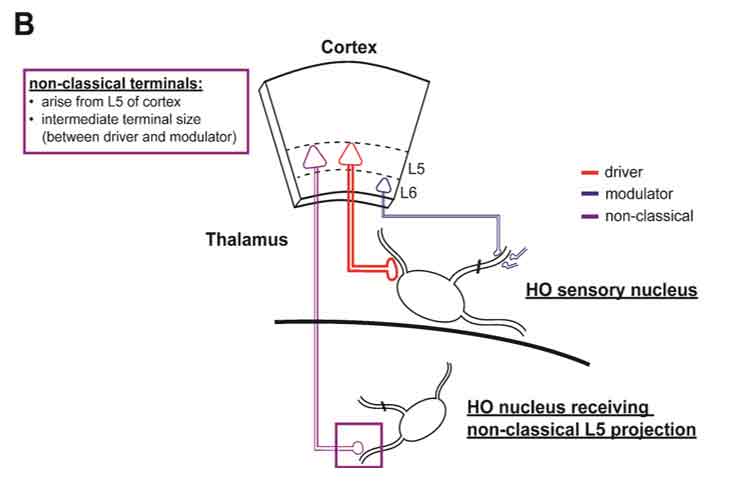
Figure 1. Drivers, modulators, and non-classical terminals.
A) Drivers and modulators as classically described in sensory thalamus. First-order (FO) thalamic nuclei receive driver inputs (red square) from subcortical sensory structures (red colored structures, left). In addition, FO nuclei, as with all thalamic nuclei, receives a L6 modulator input (blue square). Higher-order (HO) nuclei, on the other hand, receive a driver input from L5 of the visual cortex (red square), as well as a modulator input from L6 (blue square). The distinctions between classical drivers and modulators are outlined in the color-coded text boxes (top left).
B) Non-classical terminal populations arise in L5. L5 neurons in cortex also give rise to terminals smaller than classical driver populations in several thalamic nuclei. L5 produces classical driver projections in HO sensory nuclei (red), but also sends projections of intermediately-sized terminals to other HO nuclei (purple). For a full characterization of L5 terminal sizes, see (4).
induce degeneration of retinal and cortical terminal populations in LGN 13. These manipulations resulted in degeneration of large and small populations respectively, establishing a basis for recognizing these populations in electron microscopy datasets (but note a weakness in this basis that these profiles ignore other potential inputs than these two). Additional lines of evidence have since elaborated on these anatomical descriptions, demonstrating that small (presumed cortical) synapses targeted primarily distal dendrites and are more numerous than large (presumed retinal) synapses located more proximally on the dendrites or soma (14,16).
These physiological and anatomical distinctions described above demonstrate that LGN afferents from retina and L6 of cortex represent the two classes of glutamatergic inputs, drivers and modulators. Driver terminals in the retinogeniculate pathway transmit receptive fields and exhibit properties that support that function, including large terminals close to the soma that elicit large, fast postsynaptic potentials. Modulators, like L6 of cortex to LGN, appear not to transmit receptive fields and the small, weaker synapses they produce far from the cell body reflect a more nuanced function, such as gain control. (1, 10, 17). Thus, physiological and anatomical evidence support the notion that these afferent types are specialized to suit distinct functions.
Large L5 terminals drive higher-order thalamus (Figure 1A)
First-order nuclei, like LGN, represent a mere subset of the thalamus. To understand the function of remaining thalamic nuclei, a useful approach has been to identify afferents with driver properties similar to those described above for the retinogeniculate pathway. That is, identifying the nature of the information transmitted to a given thalamic nucleus (i.e., its drivers) suggests its potential function. Importantly, contemporary evidence demonstrates that in contrast to first-order nuclei, which receive driver inputs from subcortical sources, most of the thalamus instead receives driver afferents from cortical L5—these are called higher-order thalamic nuclei (2, 3, 18, 19). The broad functions of this predominant corticofugal projection have been discussed in recent reviews (3, 11, 18, 20,22).
In the visual system, L5 of primary visual cortex, which does not target the first-order LGN (a common pattern of L5 drivers), densely innervates the higher-order pulvinar nucleus (2, 23, 24). Axonal terminals from L5 of visual cortex to pulvinar exhibit similar characteristics to the retinogeniculate synapse. First, pulvinar activity is dependent on these inputs; as inactivation of visual cortex by lesion eliminates visual responses in pulvinar (25). Physiologically, these inputs exhibit synaptic properties associated with drivers: in vitro optical activation of genetically targeted L5 terminals elicits potentials recorded in pulvinar cells that are large, depressing, and lack a slow metabotropic component (23). Anatomically, L5 represents a minority of pulvinar afferents but these are large and close to soma, relative to other terminals, including those from L6 (26,33) Note that this organization functionally parallels retinal drivers and L6 cortical modulators in the first-order LGN. Therefore, although relay cells in the pulvinar receive both a driving and modulatory input from cortex, these arise from distinct L5 and L6 neuronal populations, respectively. Importantly, it has recently been demonstrated that these connectivity patterns are conserved across multiple sensory modalities, including the somatosensory system in mice (23, 34).
New evidence for diversity among L5 terminals (Figure 1B)
Because qualities associated with drivers have been observed among L5 terminals in a number of thalamic nuclei (2), projections from this layer are assumed to display a similar phenotype at all its thalamic targets. However, our lab recently demonstrated that L5 terminals may exhibit more morphological diversity than previously appreciated (4). We utilized the Rbp4-Cre mouse line (35), in which expression of Cre recombinase is restricted to cortical cells of L5, and drove Cre-dependent viral expression of the fluorescent protein EYFP to selectively label L5 axons traversing the brain, including thalamus. We labeled L5 axons in four functionally distinct cortical areas, including primary visual, primary somatosensory, primary motor and prefrontal cortex, to ensure our results reflected L5 generally rather than the specialization of a single modality (4).
First, we found the distribution of L5 terminals in thalamus to be more widespread than previously recognized. For instance, in addition to the well characterized projection to the higher-order pulvinar nucleus, visual cortex also innervated the lateral dorsal, central lateral, and intergeniculate nuclei. Likewise, somatosensory cortex innervated not only the major higher order somatosensory nucleus, the posterior medial nucleus, but also pulvinar, the lateral dorsal, central lateral, medial dorsal and parafascicular nuclei. In general, each cortical injection demonstrated clear L5 terminal fields in several additional nuclei, particularly midline nuclei, beyond the anticipated higher order nuclei (4).
Further, a detailed cataloging of the L5 terminal fields in each thalamic nucleus revealed frequent overlap of L5 projections across cortical and thalamic modalities. For instance, visual and somatosensory cortical L5 terminals overlap in several target thalamic nuclei, including the pulvinar and lateral dorsal nuclei. Intriguingly, this was true for any pair among our four cortical injection sites, suggesting several nuclei are integrating information from multiple cortical L5 projections. Of note are the medial dorsal nucleus, which was innervated by three of the four cortical areas and thought to be a hub for higher cognitive functions (36, 37) and the central lateral nucleus, which was innervated by all four cortical areas injected. This finding suggests a wide degree of convergence of corticothalamic pathways arising in L5 (4).
Qualitatively, we observed that the innervation of multiple thalamic targets by a given cortical area was not always discrete; rather, terminal fields spanned multiple thalamic targets without regard for anatomical boundaries. Given this evidence, classification of thalamic nuclei using cytoarchitectural techniques alone is likely to underappreciate nuances in the connectivity that point to the function of thalamic relay cells. Importantly, these terminal fields were non-homogenous across their extents, displaying variation in terminal density and morphology.
To objectively assess variations in terminal morphology, we quantified terminal size in different corticothalamic pathways, that is, arising from different cortical areas and/or produced in different thalamic nuclei. In an effort to categorize these L5 terminals as modulators (small terminals) or drivers (large terminals), we took advantage of the established differences between L6 modulator and L5 driver pathways in the somatosensory thalamus. Specifically, we statistically compared our measured terminal sizes across thalamic nuclei to those in the established driver (L5 to posterior medial nucleus) or modulator pathways (L6 to ventral posterior medial nucleus). Surprisingly, rather than fitting neatly into these categories, a majority of corticothalamic pathways originating in L5, including visual cortex to pulvinar, exhibited an average terminal size significantly smaller than the established large standard for drivers. Further, average terminal size was also typically larger than the established small standard for modulators. In a few pathways, however, this difference was non-significant. For example, L5 somatosensory cortex terminals in the parafascicular nucleus, though they were significantly smaller than stereotypical drivers, were not larger than the small standard established for modulator terminals, failing to distinguish them from classical modulator-type synapses (4).
Together, these findings suggest that among corticothalamic pathways, most L5 terminal populations do not exhibit an axonal terminal size that is neatly in register with either drivers or modulators. Further, they also suggest that L5 projections arising from the same cortical area (e.g. somatosensory cortex) can give rise to projections with distinct terminal properties across multiple thalamic nuclei, as is seen when comparing terminals from somatosensory cortex to posterior medial versus perifascicular nuclei 4. Thus, our recent evidence supports, at least to some degree, the suggestion by other research groups (38) calling for nuance in the driver-modulator framework that may include a non-classical terminal subtype (Figure 1B).
Considering the variation in average terminal size from L5 projections and the notion that terminal size has been linked to synaptic function (39,42), we anticipate that the morphological diversity amongst L5 projections reflects a physiological diversity, representing an intriguing topic of future experiments. Such a question might be assessed in vitro, by viral expression of the light-gated channelrhodopsin in L5 terminals. In such a configuration, terminals would be laser-activated while recording from neurons in target nuclei that display differences in L5 terminal size. Presumably, those nuclei exhibiting the largest terminals would display the largest laser-evoked excitatory post-synaptic potentials, and the most pronounced synaptic depression, which are characteristics of classical drivers, but what of smaller terminals, particularly those not significantly larger than from L6? Such nonclassical L5 populations might represent a subset of modulators akin to those from L6. Alternatively, these populations of small terminals might still give rise to a driver profile, albeit a relatively weak one, with smaller post-synaptic potentials and less pronounced depression, such that L5 includes a range of driver phenotypes.
To observe a range of driver phenotypes would beg the question of why such strong and weak drivers from L5 need coexist. In LGN, few retinal drivers target each cell, and this lack of convergence might potentiate the faithful transmission of receptive fields (1, 3). If non-classical terminal populations are indeed a weaker driver class, these might also exhibit more convergence than those classically described. Such an arrangement would allow target cells to integrate the receptive fields provided by converging drivers. Indeed, the L5 corticothalamic pathways we screened produced overlapping terminals especially in nuclei dealing with coding multi-sensory or associative information (i.e.., midline nuclei such as medial dorsal nucleus). Thus, we posit diversity among its L5 afferents reflects the computational richness of thalamic coding.
References
1. S. M. Sherman, R. W. Guillery, On the actions that one nerve cell can have on another: Distinguishing "drivers" from "modulators." Proc National Acad Sci 95, 7121-7126 (1998).
https://doi.org/10.1073/pnas.95.12.7121
PMid:9618549 PMCid:PMC22761
2. S. M. Sherman, R. W. Guillery, Exploring the Thalamus and Its Role in Cortical Function (2009) https:/doi.org/10.7551/mitpress/2940.003.0008.
https://doi.org/10.7551/mitpress/2940.001.0001
3. S. M. Sherman, The thalamus is more than just a relay. Curr Opin Neurobiol 17, 417-22 (2007).
https://doi.org/10.1016/j.conb.2007.07.003
PMid:17707635 PMCid:PMC2753250
4. J. A. Prasad, B. J. Carroll, S. M. Sherman, Layer 5 Corticofugal Projections from Diverse Cortical Areas: Variations on a Pattern of Thalamic and Extrathalamic Targets. J Neurosci Official J Soc Neurosci 40, 5785-5796 (2020).
https://doi.org/10.1523/JNEUROSCI.0529-20.2020
PMid:32532890 PMCid:PMC7380964
5. W. M. Usrey, J. B. Reppas, R. C. Reid, Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature 395, 384-387 (1998).
https://doi.org/10.1038/26487
PMid:9759728
6. E. Kaplan, R. Shapley, The origin of the S (slow) potential in the mammalian Lateral Geniculate Nucleus. Exp Brain Res 55, 111-116 (1984).
https://doi.org/10.1007/BF00240504
PMid:6086369
7. T. Tsumoto, O. D. Creutzfeldt, C. R. Legéndy, Functional organization of the corticofugal system from visual cortex to lateral geniculate nucleus in the cat. Exp Brain Res 32, 345-364 (1978).
https://doi.org/10.1007/BF00238707
PMid:210031
8. R. E. Kalil, R. Chase, Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol 33, 459-474 (1970).
https://doi.org/10.1152/jn.1970.33.3.459
PMid:4314735
9. H. E. Jones, et al., Differential Feedback Modulation of Center and Surround Mechanisms in Parvocellular Cells in the Visual Thalamus. J Neurosci 32, 15946-15951 (2012).
https://doi.org/10.1523/JNEUROSCI.0831-12.2012
PMid:23136432 PMCid:PMC6621639
10. W. Wang, I. M. Andolina, Y. Lu, H. E. Jones, A. M. Sillito, Focal Gain Control of Thalamic Visual Receptive Fields by Layer 6 Corticothalamic Feedback. Cereb Cortex 28, 267-280 (2016).
https://doi.org/10.1093/cercor/bhw376
PMid:27988493
11. W. M. Usrey, S. M. Sherman, Corticofugal circuits: Communication lines from the cortex to the rest of the brain. J Comp Neurol 527, 640-650 (2019).
https://doi.org/10.1002/cne.24423
PMid:29524229 PMCid:PMC6131091
12. D. A. McCormick, M. von Krosigk, Corticothalamic activation modulates thalamic firing through glutamate "metabotropic" receptors. Proc National Acad Sci 89, 2774-2778 (1992).
https://doi.org/10.1073/pnas.89.7.2774
PMid:1313567 PMCid:PMC48745
13. J. Szentágothai, J. Hámori, T. Tömböl, Degeneration and electron microscope analysis of the synaptic glomeruli in the lateral geniculate body. Exp Brain Res 2, 283-301 (1966).
https://doi.org/10.1007/BF00234775
PMid:5957903
14. R. W. Guillery, The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie 96, 1-38 (1969).
https://doi.org/10.1007/BF00321474
https://doi.org/10.1007/BF00321475
PMid:5772028
15. V. M. Montero, A quantitative study of synaptic contacts on interneurons and relay cells of the cat lateral geniculate nucleus. Exp Brain Res 86, 257-270 (1991).
https://doi.org/10.1007/BF00228950
PMid:1756802
16. J. R. Wilson, N. Bose, S. M. Sherman, Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc Royal Soc Lond Ser B Biological Sci 221, 411-436 (1984).
https://doi.org/10.1098/rspb.1984.0042
PMid:6146984
17. S. R. Olsen, D. S. Bortone, H. Adesnik, M. Scanziani, Gain control by layer six in cortical circuits of vision. Nature 483, 47-52 (2011).
https://doi.org/10.1038/nature10835
PMid:22367547 PMCid:PMC3636977
18. M. S. Sherman, Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 19, 533-541 (2016).
https://doi.org/10.1038/nn.4269
PMid:27021938
19. S. M. Sherman, R. W. Guillery, Functional organization of thalamocortical relays. J Neurophysiol 76, 1367-1395 (1996).
https://doi.org/10.1152/jn.1996.76.3.1367
PMid:8890259
20. M. S. Sherman, Thalamocortical interactions. Curr Opin Neurobiol 22, 575-579 (2012).
https://doi.org/10.1016/j.conb.2012.03.005
PMid:22498715 PMCid:PMC3398163
21. M. S. Sherman, R. W. Guillery, Functional Connections of Cortical Areas (2013) https:/doi.org/10.7551/mitpress/9780262019309.001.0001.
https://doi.org/10.7551/mitpress/9780262019309.001.0001
22. W. M. Usrey, S. M. Sherman, Exploring Thalamocortical Interactions: Circuitry for Sensation, Action, and Cognition (Oxford University Press, 2021) https://doi.org/10.1093/med/9780197503874.001.0001
23. A. J. Miller-Hansen, S. M. Sherman, Conserved patterns of functional organization between cortex and thalamus in mice. Proc National Acad Sci 119, e2201481119 (2022).
https://doi.org/10.1073/pnas.2201481119
PMid:35588455
24. A. Blot, et al., Visual intracortical and transthalamic pathways carry distinct information to cortical areas. Neuron 109, 1996-2008.e6 (2021).
https://doi.org/10.1016/j.neuron.2021.04.017
PMid:33979633 PMCid:PMC8221812
25. D. B. Bender, Visual activation of neurons in the primate pulvinar depends on cortex but not colliculus. Brain Res 279, 258-261 (1983).
https://doi.org/10.1016/0006-8993(83)90188-9
26. K. S. Rockland, Two types of corticopulvinar terminations: Round (type 2) and elongate (type 1). J Comp Neurol 368, 57-87 (1996).
https://doi.org/10.1002/(SICI)1096-9861(19960422)368:1<57::AID-CNE5>3.0.CO;2-J
27. K. S. Rockland, Further evidence for two types of corticopulvinar neurons. Neuroreport 5, 1865-1868 (1994).
https://doi.org/10.1097/00001756-199410000-00006
PMid:7841364
28. K. S. Rockland, Convergence and branching patterns of round, type 2 corticopulvinar axons. J Comp Neurol 390, 515-536 (1998).
​https://doi.org/10.1002/(SICI)1096-9861(19980126)390:4<515::AID-CNE5>3.0.CO;2-3
29. J. Bourassa, M. Descheˆnes, Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience 66, 253-263 (1995).
https://doi.org/10.1016/0306-4522(95)00009-8
30. H. Ojima, K. Murakami, K. Kishi, Dual termination modes of corticothalamic fibers originating from pyramids of layers 5 and 6 in cat visual cortical area 17. Neurosci Lett 208, 57-60 (1996).
https://doi.org/10.1016/0304-3940(96)12538-6
31. C. Darianâ€Smith, A. Tan, S. Edwards, Comparing thalamocortical and corticothalamic microstructure and spatial reciprocity in the macaque ventral posterolateral nucleus (VPLc) and medial pulvinar. J Comp Neurol 410, 211-234 (1999).
https://doi.org/10.1002/(SICI)1096-9861(19990726)410:2<211::AID-CNE4>3.0.CO;2-X
32. S. Wang, M. A. Eisenback, M. E. Bickford, Relative distribution of synapses in the pulvinar nucleus of the cat: Implications regarding the "driver/modulator" theory of thalamic function. J Comp Neurol 454, 482-494 (2002).
https://doi.org/10.1002/cne.10453
PMid:12455011
33. Z. Vidnyánszky, Z. Borostyánkõi, T. J. Görcs, J. Hámori, Light and electron microscopic analysis of synaptic input from cortical area 17 to the lateral posterior nucleus in cats. Exp Brain Res 109, 63-70 (1996).
https://doi.org/10.1007/BF00228627
PMid:8740209
34. C. Mo, I. Petrof, A. N. Viaene, S. M. Sherman, Synaptic properties of the lemniscal and paralemniscal pathways to the mouse somatosensory thalamus. Proc National Acad Sci 114, E6212-E6221 (2017).
https://doi.org/10.1073/pnas.1703222114
PMid:28696281 PMCid:PMC5544298
35. C. R. Gerfen, R. Paletzki, N. Heintz, GENSAT BAC Cre-Recombinase Driver Lines to Study the Functional Organization of Cerebral Cortical and Basal Ganglia Circuits. Neuron 80, 1368-1383 (2013).
https://doi.org/10.1016/j.neuron.2013.10.016
PMid:24360541 PMCid:PMC3872013
36. G. Pergola, et al., The Regulatory Role of the Human Mediodorsal Thalamus. Trends Cogn Sci 22, 1011-1025 (2018).
https://doi.org/10.1016/j.tics.2018.08.006
PMid:30236489 PMCid:PMC6198112
37. A. S. Mitchell, S. Chakraborty, What does the mediodorsal thalamus do? Frontiers Syst Neurosci 7, 37 (2013).
https://doi.org/10.3389/fnsys.2013.00037
PMid:23950738 PMCid:PMC3738868
38. M. E. Bickford, Thalamic Circuit Diversity: Modulation of the Driver/Modulator Framework. Front Neural Circuit 9, 86 (2016).
https://doi.org/10.3389/fncir.2015.00086
PMid:26793068 PMCid:PMC4709853
39. I. Petrof, S. M. Sherman, Functional significance of synaptic terminal size in glutamatergic sensory pathways in thalamus and cortex. J Physiology 591, 3125-3131 (2013).
https://doi.org/10.1113/jphysiol.2012.247619
PMid:23359668 PMCid:PMC3717215
40. Y. Geinisman, Perforated axospinous synapses with multiple, completely partitioned transmission zones: Probable structural intermediates in synaptic plasticity. Hippocampus 3, 417-433 (1993).
https://doi.org/10.1002/hipo.450030404
PMid:8269034
41. N. Holderith, et al., Erratum: Corrigendum: Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci 19, 172-172 (2016).
https://doi.org/10.1038/nn0116-172
PMid:26713747
42. J. Matz, A. Gilyan, A. Kolar, T. McCarvill, S. R. Krueger, Rapid structural alterations of the active zone lead to sustained changes in neurotransmitter release. Proc National Acad Sci 107, 8836-8841 (2010).
https://doi.org/10.1073/pnas.0906087107
PMid:20421490 PMCid:PMC2889309
43. W. M. Usrey, J. B. Reppas, R. C. Reid, Specificity and Strength of Retinogeniculate Connections. J Neurophysiol 82, 3527-3540 (1999).
https://doi.org/10.1152/jn.1999.82.6.3527
PMid:10601479